Cells make decisions by responding to their environment through activation of dynamic signaling networks . We build molecular tools to understand this “signaling code”, to understand how it changes in disease, and to generate new methods for remote control of cell function.
Research
New molecular tools for precision remote control of cells
Our lab specializes in optogenetic and, more recently, thermogenetic technologies, which let us control cellular events in real-time using light or temperature as stimuli of cell functions. These technologies are powerful because, unlike more traditional approaches, they can perturb cells with the dynamics and high resolution in space and time that naturally regulate cell behavior.
Optogenetics
Why? Light-activation lets us arbitrarily define the intensity, timing, and location of signals within a cell, with unmatched speed (seconds) and precision (um-scale). These probes are ideal to mimic a cell’s dynamic signaling environment and to systematically explore the effects of these important signal parameters. And because light is programmable and miniaturizable, we can scale and automate optogenenetics experiments for systematic, robust, yet straightforward studies.
How? We engineer optogenetic proteins by borrowing from plants, which evolved light-sensitive proteins to sense and respond to sunlight. These proteins perform a variety of useful light-responsive behaviors, for example dimerization, aggregation, or relocation to the cell membrane. We use protein engineering to couple these behaviors to cell signaling proteins such that light triggers activity of these proteins and their networks.
Left: Light-induced clustering of Cry2 in mammalian cells
Right: Light-induced membrane translocation in a zebrafish embryo.
Thermogenetics
Why? Optical control is powerful, but light can’t penetrate human tissues effectively and thus is poorly suited for animal studies or potential therapies. Thermal control offers a more penetrant alternative, since temperature can be controlled non-invasively in tissues with millimeter-scale precision up to ~10 cm deep (compared to ~1 mm for blue light). However, there arecurrently few known thermally-sensitive proteins that could be applied for this purpose. We are engineering modular thermally-responsive proteins, with the goal of controlling a diverse array of cell and protein behaviors with temperature in the same manner as is currently possibly with optogenetics. These technologies could ultimately be be applied to generate more effective and safer engineered cell therapies.
How? Our lab recently discovery that a fungal protein called BcLOV4 can change its activity in response to both light and temperature. We have engineered variants of this protein that respond only to temperature. We continue to engineer and optimize its properties and to embed it within cellular networks that control a variety of cell behaviors, including those relevant for control of cell therapies.
Top: A thermally-sensitive protein resides at the membrane but dissociates at elevated temperatures
Bottom: Reversible translocation from the membrane to the nucleus in response to temperature changes.
Signal (mis)perception in cancer and drug resistance
Cancer is not strictly a cell-autonomous disease, but rather depends on how cancer cells sense and escape proper regulation by their environment. Similarly, the cancer cell environment shapes the extent to which cancer cells respond to or resist therapy. We use optogenetics and live-cell microscopy to explore the hypothesis that cancer mutations can fundamentally alter a cell’s perception of its environment, leading to pathological cell decisions like uncontrolled growth or survival in the presence of drugs designed to kill the cell. Our work has discovered oncogene-associated changes in dynamic responses in proliferative signaling, as well as oncogene-mediated sensitization of drug resistance signaling. We aim to better understand oncogenesis and drug resistance, allowing us to identify new disease metrics and treatment strategies .
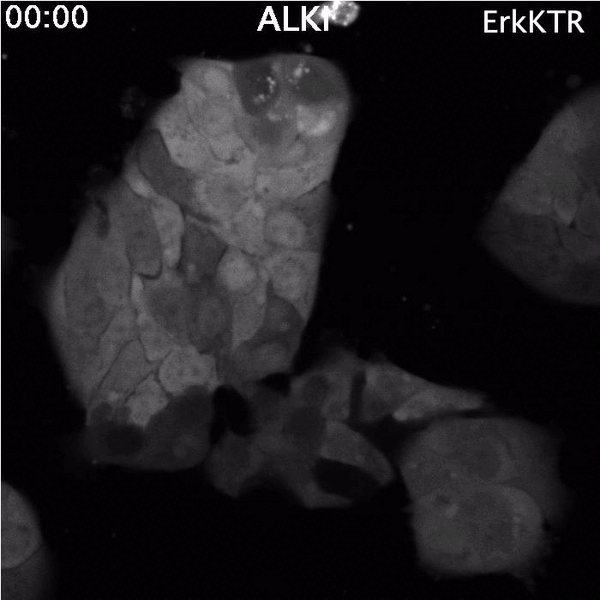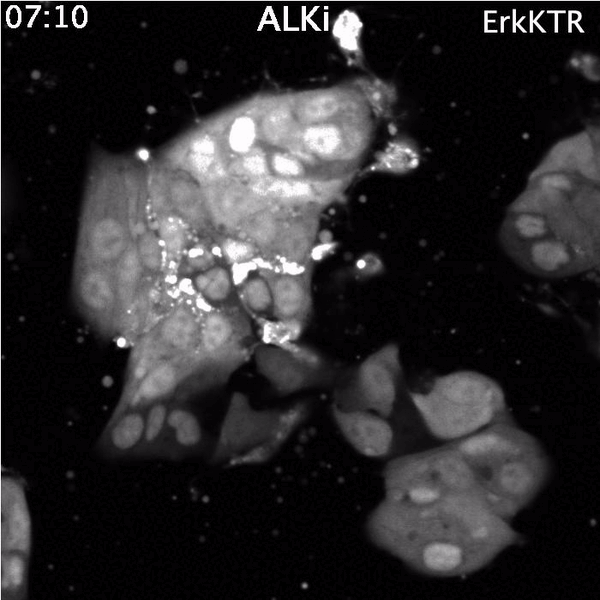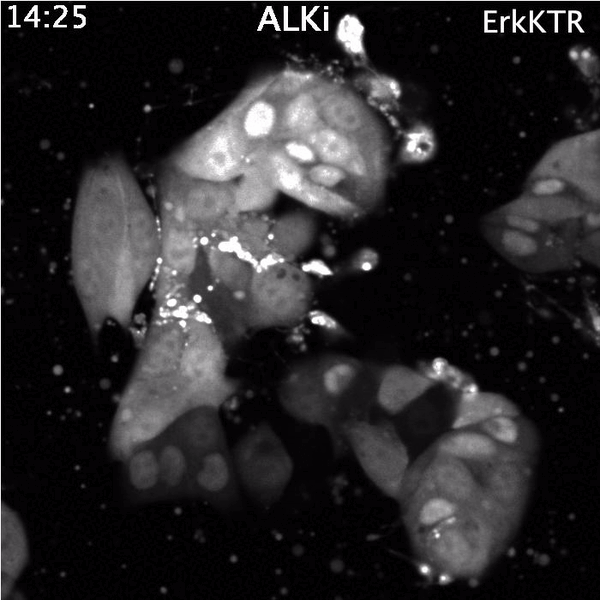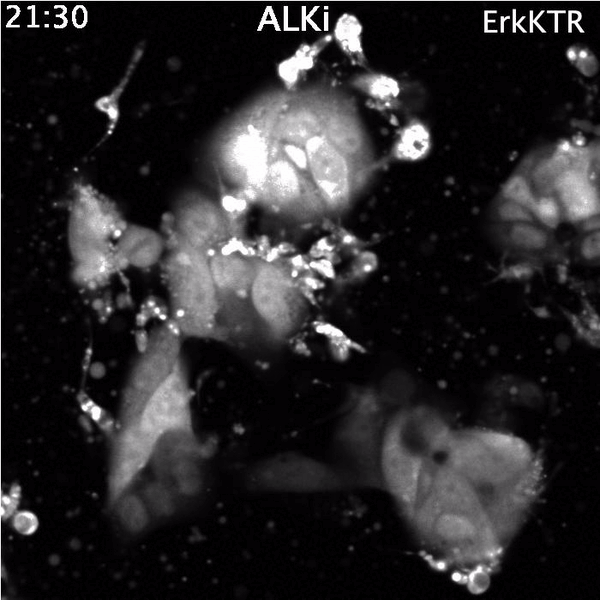
Top: Spontaneous Erk reactivation in cancer cells treated with a targeted therapy. Cells express the ErkKTR reporter.
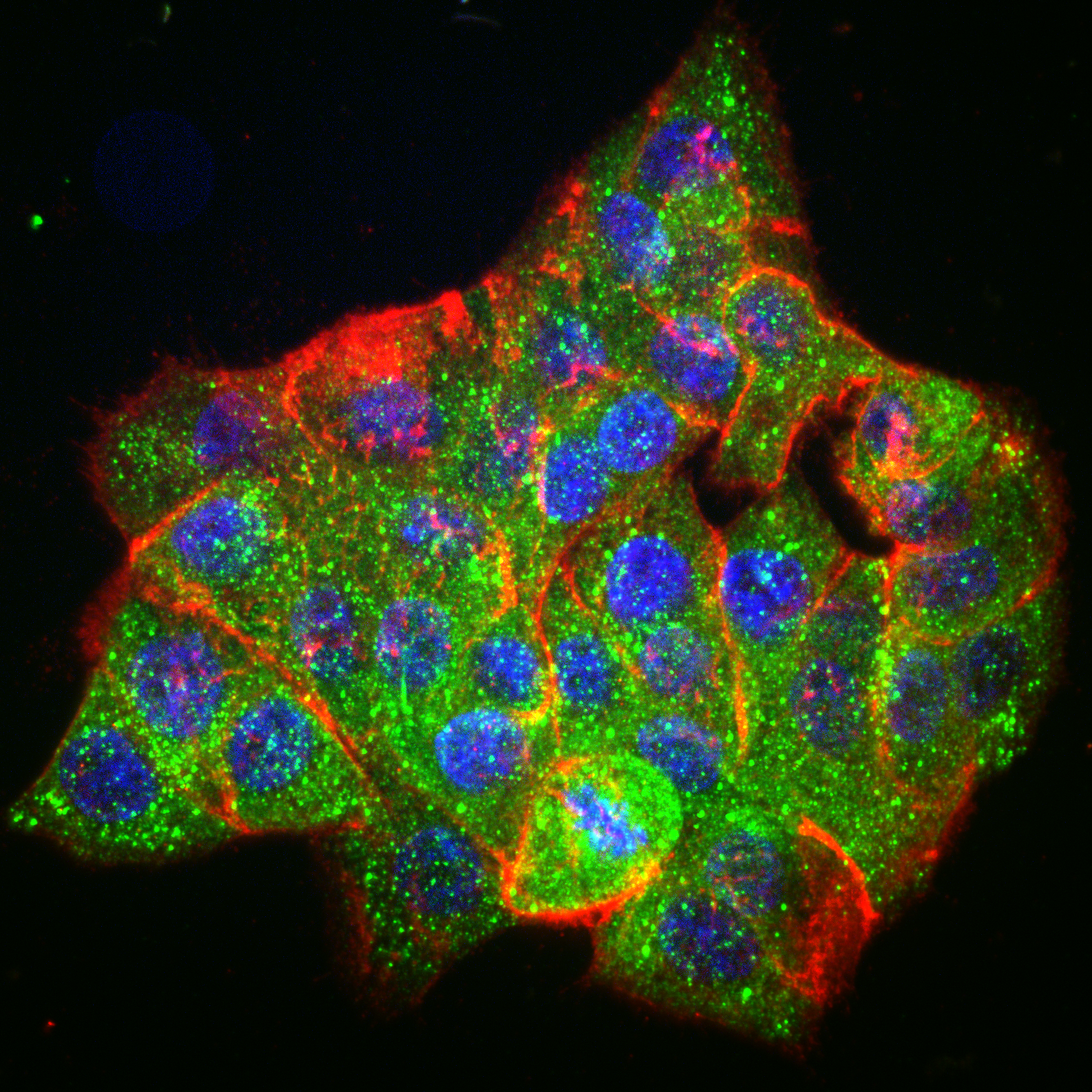
Bottom: Lung cancer cells driven by EML4-ALK, immunostained for EML4-ALK (green), EGFR (red) and DNA (blue)
Visualizing “invisible” protein aggregates
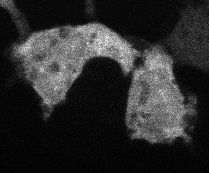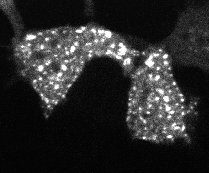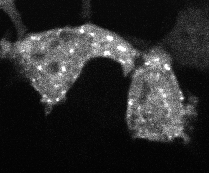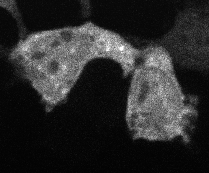
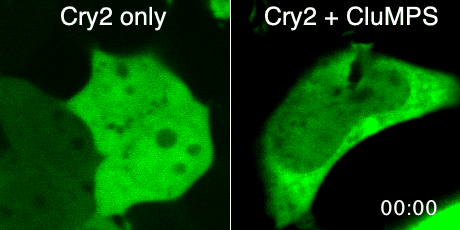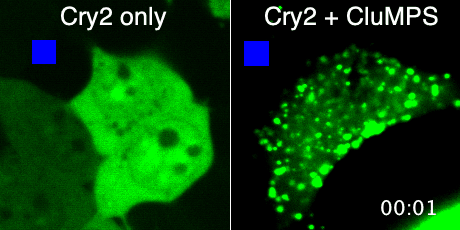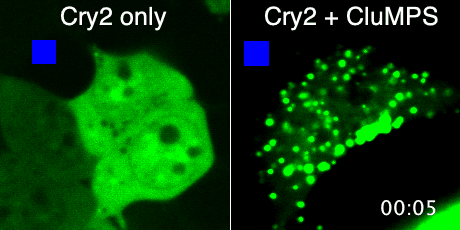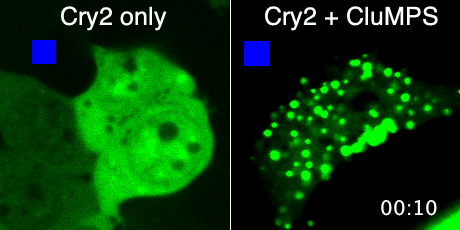
Protein aggregation is a versatile way in which protein activity is controlled within the cell. However, we don’t know the extent to which protein clustering occurs because we often can’t observe such clusters in cells. Limitations in standard fluorescence microscopy only allow us to visualize clusters that are greater than ~tens of monomers in size, almost an order of magnitude greater than many physiological clusters. We developed a new reporter strategy to address this gap, called CluMPS. CluMPS amplifies small clusters so that they are readily visible. We are applying this technology to visualize the prevalence, context, and function of protein clustering throughout the proteome. We expect this work will allow us to observe and control biological regulation in new and impactful ways.
Top: The CluMPS reporter strategy
Bottom: The CluMPS amplifies small clusters of Cry2 that otherwise would not be visible
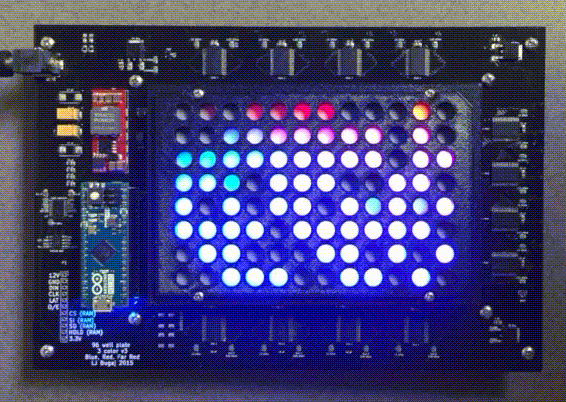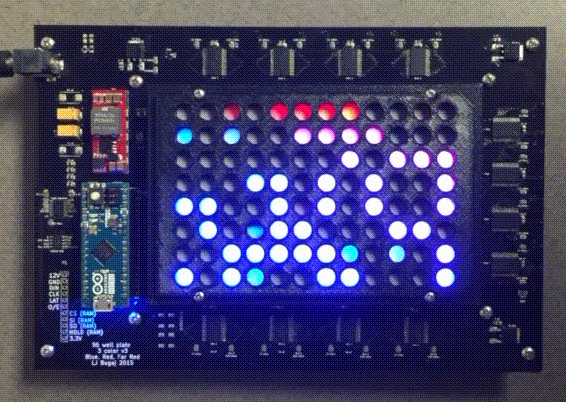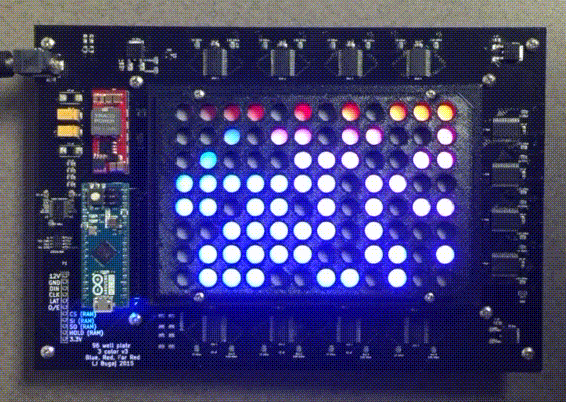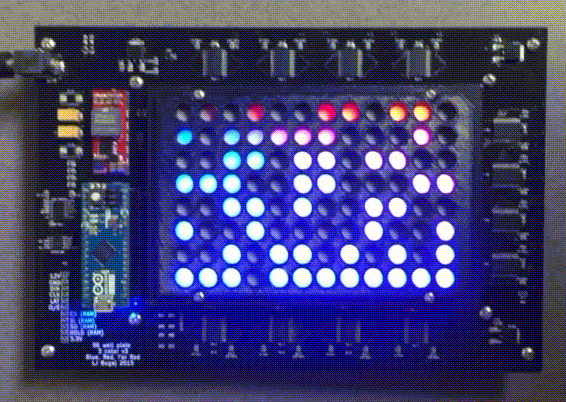
Custom instrumentation for remote control of cells
Because light and temperature are non-invasive stimuli and can be generated with semiconductors, there is tremendous potential to miniaturize, parallelize, and scale up optogenetic and thermogenetic experiments. Our lab develop custom open-source devices for this purpose. We design our devices so that they can be easily shared, built, and used by labs around the world. The high-throughput, programmable experiments enabled by these devices allows robust and systematic exploration of large perturbation spaces in an easy, automated fashion.
The optoPlate